Explore 7 groundbreaking breakthroughs in next-gen bioelectronics, merging biology with circuits. Discover living electronics, biohybrid sensors, electrogenetics, and the future of medicine and robotics.
Next-Gen bioelectronics is bridging the gap between living systems and human-made circuits by embedding electronics into biological environments and engineering cells to communicate with devices. Through innovations in materials, synthetic biology, and interface engineering, this field unlocks new paradigms in health monitoring, therapies, soft robotics, and smart biosensing.
Introduction: Why “Bio + Electronics” Matters Now
Biological systems naturally operate with ions, electrons, chemical messengers, and fields. Traditional electronics, on the other hand, use solid-state semiconductors and wires. The challenge—and the opportunity—is to bring those two worlds into seamless integration.
This merging has given rise to bioelectronics, biohybrid systems, and living electronics. As of 2025, these are not just science-fiction fantasies: researchers are designing devices that interface with cells, tissues, or even entire organs. (Cell Reports Physical Science)
If done well, next-gen bioelectronics could yield diagnostics that sense molecular changes in your body in real time, implants that heal or augment function, and hybrid robots with living components. But to get there, we must solve deep challenges of biocompatibility, interface stability, power, control, and integration.
In this article, you’ll get:
- A clear grounding in fundamentals.
- Vivid real-life examples being built or prototyped today.
- Answers to trending FAQs.
- Guidance on SEO optimization with WordPress + Rank Math.
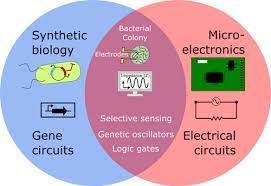
What Is the Intersection of Biology and Electronics?
The intersection of biology and electronics lies in the field of bioelectronics—where living systems and engineered circuits exchange signals, energy, and information.
Biological systems naturally use ions, proteins, and chemical gradients to transmit information, while electronics rely on electrons flowing through semiconductors and circuits. Bridging these two “languages” allows scientists to create hybrid devices that can:
- Sense biological processes in real time (e.g., glucose monitors, neurotransmitter sensors).
- Stimulate or modulate biological activity (e.g., pacemakers, electrogenetic implants).
- Enable adaptive, closed-loop therapies.
- Create biohybrid robots where living cells provide computation or movement, while circuits coordinate activity.
Example: MIT researchers developed a swallowable capsule housing engineered bacteria that sense gut inflammation. When triggered, the bacteria signal to embedded microelectronics, which then transmit wireless data to a smartphone app. This is a literal merging of biological sensing and electronic processing.
1. Foundations: What Is Next-Gen Bioelectronics?
1.1 Core Definitions
- Bioelectronics: Devices or systems that interface with living biological systems.
- Biohybrid systems: Combine living components with nonliving devices. (MIT Galloway Lab)
- Living electronics / synthelectronics: Genetically engineered organisms act as integral parts of circuits.
- Organic Electrochemical Transistors (OECTs): Transistors compatible with ionic environments.
1.2 Why Now?
Key drivers include:
- Biomaterials advances like hydrogels and soft polymers.
- Synthetic biology enabling engineered cellular behavior.
- Low-power microelectronics for implantable systems.
- Electrogenetics, where electricity controls gene expression.
- Demand from healthcare and robotics for adaptive devices.
What Are the Five Main Types of Circuits?
Whether in bioelectronics or traditional systems, circuits form the backbone of devices. The five main types are:
- Series Circuit – Single path; if one element fails, the circuit breaks.
- Example: Old holiday lights.
- Parallel Circuit – Multiple paths; failure of one branch doesn’t stop others.
- Example: Household wiring.
- Combination Circuit – Mix of series and parallel elements.
- Example: Computer motherboards.
- Integrated Circuits (ICs) – Miniaturized circuits on silicon chips.
- Example: Wearables or bioelectronic implants.
- Printed Circuit Boards (PCBs) – Platforms connecting components with conductive tracks.
- Example: Biosensors and pacemakers.
In bioelectronics, circuits often use soft ionic conductors instead of rigid silicon, making them more compatible with living tissue.
2. How Do Biology and Circuits Actually Interface?
To function, bioelectronic systems need bi-directional signal transfer.
- Ionic–Electronic Interfaces: Convert ionic currents to electronic currents.
- Electrochemical Signaling: Electrodes sense neurotransmitters or metabolites.
- Electrical Stimulation: Classic pacemaker model, now extended to electrogenetics.
- Optical Interfaces: Optogenetics and photovoltaic systems.
- Mechanical & Thermal Interfaces: Trigger mechanosensitive or thermogenetic circuits.
Hierarchy: Modern systems often layer modalities—e.g., electrodes trigger gene circuits, leading to chemical or electrical feedback.
3. Real-World Examples & Use Cases
- Living Bacterial Biosensors: Detect gut inflammation inside capsules.
- Electrogenetic Therapy: Cells engineered to produce insulin when stimulated by a small implant.
- Neuromorphic Soft Electronics: Circuits mimicking neurons that integrate with living tissue.
- Photovoltaic Bioelectronics: Artificial retinas using light-sensitive semiconductors.
- Hydrogel Interfaces: Soft, hydrated connections that reduce rejection.
(FAQs)
Q1: What are “living bioelectronics”?
Living bioelectronics refer to systems where engineered living cells or tissues actively become part of a circuit rather than being just external targets. Instead of simply being stimulated by electrodes, these cells can sense, compute, adapt, and output signals as if they were components of an electronic device.
For example, researchers at MIT and Harvard have engineered bacteria to sense toxins in the environment. When they detect a target molecule, they produce an electrical current or fluorescence that interfaces with microelectronic circuits. This transforms living organisms into biological sensors that communicate directly with electronics.
The advantage here is adaptability—living systems can self-repair, respond dynamically to their environments, and evolve functionality over time, unlike rigid hardware. In the future, we may see “biochips” made not just from silicon, but from microbial colonies genetically programmed to process data in real-time.
In short, living bioelectronics represent a paradigm shift: from using devices to control biology, to integrating biology itself as part of the circuitry.
Q2: How do you prevent fibrosis around implants?
Fibrosis is the body’s natural reaction to foreign materials. When an implant is placed inside the body, immune cells often form scar tissue around it, which can reduce its effectiveness by blocking signals or limiting integration. Preventing fibrosis is one of the greatest challenges in long-term bioelectronics.
Scientists are tackling this issue with several strategies:
- Hydrogel coatings: These water-rich, soft materials mimic the consistency of living tissue, reducing the “foreign body” response.
- Anti-fouling surfaces: Special coatings prevent protein buildup and immune cell attachment.
- Biomimetic textures: Micro- and nano-scale patterns on implant surfaces encourage cells to accept the material as “natural.”
- Drug-eluting implants: Devices that slowly release anti-inflammatory agents can calm immune responses over time.
Real-world example: Researchers working on neural implants for Parkinson’s disease use hydrogel-wrapped electrodes to maintain long-term stability. Without these measures, signal quality drops dramatically as scar tissue builds up.
In short, the key to preventing fibrosis is making implants look, feel, and act more like natural tissue, reducing the body’s urge to wall them off.
Q3: Are there clinical uses?
Yes, bioelectronics already have major clinical applications—and many more are in development. Some are widely used in medicine today:
- Cochlear implants restore hearing by bypassing damaged ear cells and stimulating the auditory nerve.
- Vagus nerve stimulators are FDA-approved for epilepsy and depression.
- Cardiac pacemakers and defibrillators are classic bioelectronic devices that regulate heart rhythm.
- Retinal prosthetics help partially restore vision in patients with certain types of blindness.
Beyond current devices, experimental biohybrid systems are emerging. For instance, insulin-producing cells can be engineered to release insulin in response to wireless electronic stimulation, potentially revolutionizing diabetes management. Similarly, brain-computer interfaces (BCIs) are being tested to help paralyzed individuals regain movement.
The clinical promise of bioelectronics lies in closed-loop therapies—devices that sense a biological signal, process it, and deliver a corrective response instantly. Instead of taking drugs with unpredictable side effects, patients could one day have self-regulating implants powered by bioelectronics.
Q4: What is electrogenetics?
Electrogenetics is a groundbreaking field that combines electricity and genetic control. It refers to using electrical signals to turn genes on or off in living cells, essentially letting scientists or devices wirelessly control biological processes.
Here’s how it works: engineered cells are given synthetic gene circuits that respond to electrical inputs. When an electrode or implanted device sends a small current, these genes activate (or deactivate), triggering specific cellular behaviors like protein production.
Imagine an implant that senses rising blood sugar and sends a mild electrical pulse to engineered cells in your pancreas. Those cells could then produce insulin instantly, eliminating the need for daily injections.
This technology is still in the experimental phase, but proof-of-concept studies have been published in Nature Communications and other journals. If matured, electrogenetics could usher in a new era of precision, wireless, gene-based medicine.
Q5: Why are Organic Electrochemical Transistors (OECTs) important?
OECTs are a special kind of transistor designed to work in wet, ionic environments—exactly the conditions found in biological systems. Traditional silicon transistors fail when exposed to fluids and ions, but OECTs thrive in them.
They use conductive polymers instead of rigid silicon. When ions from a biological fluid (like blood or cerebrospinal fluid) enter the polymer, they modulate its conductivity. This allows OECTs to translate biological signals (ionic currents) into electronic ones seamlessly.
Why is this important?
- They are soft and flexible, minimizing tissue damage.
- They have low power requirements, ideal for implants.
- They enable high sensitivity biosensing, detecting tiny changes in biological activity.
Practical example: OECT-based sensors have been tested for real-time brain activity monitoring in animals, offering smoother and longer-lasting integration than traditional metal electrodes.
In essence, OECTs are the translators between biology’s ionic language and electronics’ digital one.
Q6: Can bioelectronics compute?
Yes—bioelectronics can compute, though not in the same way silicon chips do. Instead of binary transistors, bioelectronic computing uses neuromorphic principles or biological logic circuits.
- Neuromorphic devices mimic the firing patterns of neurons, using flexible materials that behave like synapses.
- Biological computing uses engineered cells that process information chemically or electrically.
For instance, scientists have demonstrated bacterial colonies that can perform logic functions (like AND/OR gates). When linked to microelectronics, these colonies act like programmable “wetware processors.”
Although we’re far from bioelectronic laptops, these systems could power adaptive implants, environmental biosensors, and robotic controls that adjust in real time based on complex biological feedback.
The future may see hybrid computing—where silicon processors handle raw speed, while living circuits handle adaptation, resilience, and biochemical context.
Q7: What are the main hurdles in developing bioelectronics?
Despite enormous progress, several hurdles stand between today’s prototypes and tomorrow’s practical devices:
- Biocompatibility: Ensuring implants don’t trigger immune rejection.
- Mechanical mismatch: Electronics are rigid; tissues are soft and dynamic. Bridging this gap is vital.
- Stability: Many bioelectronic devices degrade in wet environments.
- Power supply: Developing safe, long-lasting, and wireless power for implants is a challenge.
- Data security: Streaming biological data raises privacy and ethical concerns.
- Regulation: Biohybrid devices blur the line between medicine and biotechnology, complicating FDA approval.
Real-world case: Early brain-computer interfaces failed due to scar tissue buildup and electrode degradation. Only with advances in hydrogels and OECTs has long-term stability improved.
These challenges are not insurmountable. As materials science, synthetic biology, and microelectronics advance together, the barriers are shrinking.
Q8: How is light used in bioelectronics?
Light plays a powerful role in bioelectronics through two main approaches:
- Optogenetics: Genetically modifying cells (often neurons) to respond to light. By shining light of specific wavelengths, scientists can activate or silence targeted brain circuits. This has been used in animal models to study memory, addiction, and movement disorders.
- Photovoltaic interfaces: Using light-sensitive semiconductors to generate currents in response to light. Artificial retinas are a perfect example, where photovoltaic devices restore partial sight by converting light into electrical signals for the brain.
The beauty of light is its precision. It can target single cells or regions without invasive electrodes. However, delivering light deep into tissues requires innovations like implantable LEDs or fiber optics.
Overall, light-based bioelectronics could transform neuroscience, vision restoration, and even non-invasive therapies for psychiatric disorders.
Q9: Will bioelectronics merge with AI and IoT?
Absolutely—and this is where the field’s full potential will unfold. Bioelectronics generate massive amounts of real-time biological data (like glucose levels, brain signals, or gut metabolites). AI and IoT provide the tools to analyze, interpret, and act on that data.
Here’s the likely roadmap:
- Wearable and implantable sensors continuously stream data.
- IoT networks transmit this data securely to cloud servers.
- AI algorithms analyze patterns and make predictions—like predicting a seizure before it happens.
- Closed-loop systems send corrective signals back to the bioelectronic device, delivering instant therapy.
Imagine a pacemaker that not only regulates heartbeat but also predicts cardiac events using AI. Or an insulin implant that preemptively adjusts blood sugar levels based on machine learning forecasts.
The convergence of bioelectronics + AI + IoT will define the next decade of digital health and human augmentation.
6. Strategic Takeaways
- Bioelectronics is shifting from theory to practice.
- It holds promise in medicine, robotics, and AI-powered IoT.
- Soft materials, electrogenetics, and neuromorphic circuits are key trends.
- Blogging about it requires clarity, depth, and authority to rank high.
Final Thoughts
Next-Gen bioelectronics is not a distant dream but a rapidly maturing discipline. From ingestible biosensors to neuromorphic implants, the union of biology and circuits is paving the way toward smarter healthcare, adaptive robotics, and even AI-driven living electronics.
For bloggers, content creators, and innovators, now is the right time to capture attention by educating readers and building authority around this revolutionary field.